Abstract
Background: Invasive fungal diseases (IFDs), such as invasive mucormycosis (IM) and invasive aspergillosis (IA), are life-threatening infections that remain a major cause of morbidity and mortality in a wide range of immunocompromised patients, including older individuals. During the COVID-19 pandemic, a rise in the proportion of older patients with IFDs was observed. Isavuconazole is a broad-spectrum triazole, approved for the treatment of IM and IA in adults. Here, we conducted a post-hoc analysis of two phase 3 studies to evaluate the safety and efficacy of isavuconazole specifically in patients ≥65 years with IFDs.
Methods: Data from two prospective clinical trials, VITAL (NCT00634049) and SECURE (NCT00412893), were included. VITAL was a single-arm, open-label study evaluating isavuconazole for rare IFDs (mainly IM) and IA in renally-impaired patients. SECURE was a randomized, double-blind, non-inferiority study comparing isavuconazole with voriconazole for IA. Safety (adverse events [AEs] by system organ class) and efficacy (all-cause mortality through day 42 and overall responses at end of treatment [EOT]) were assessed for the ≥65 and <65 years subgroups.
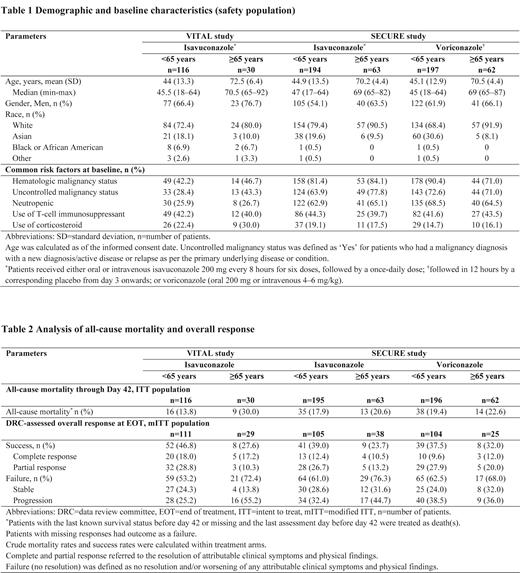
Results: At enrollment, 116/146 patients were <65 years and 30/146 patients were ≥65 years in VITAL, and 391/516 patients were <65 years and 125/516 patients were ≥65 years in SECURE. Baseline characteristics were mostly comparable for both subgroups across both studies and between the isavuconazole and voriconazole treatment arms in SECURE (Table 1).
Safety
In VITAL, AEs were reported in similar proportions in both subgroups (≥65 years: 100.0%; <65 years: 94.0%); with infections and infestations (≥65 years: 63.3%; <65 years: 62.1%) being the most common. Serious AEs (SAEs) were higher in the ≥65 years (76.7%) compared to the <65 years (56.9%) subgroup, although drug-related AEs had a reverse trend (≥65 years: 33.3%, <65 years: 43.1%). The most common drug-related AEs were gastrointestinal disorders (≥65 years: 13.3%; <65 years: 16.4%).
In SECURE, a similar proportion of AEs was reported in both subgroups of the isavuconazole arm (≥65 years: 98.4%; <65 years: 95.4%), with gastrointestinal disorders (≥65 years: 73.0% <65 years: 66.0%) being the most common. SAEs were reported at higher rates (61.9% vs 49.0%) in the ≥65 years than the <65 years subgroup in the isavuconazole arm. Drug-related AEs were reported more frequently in both subgroups of the voriconazole arm (≥65 years: 54.8%; <65 years: 61.4%) than the isavuconazole arm (≥65 years: 44.4%; <65 years: 41.8%). Additionally, in the ≥65 years subgroup, a higher proportion of patients (cut-off difference: ≥5%) experienced drug-related cardiac disorders (isavuconazole: 6.3%; voriconazole: 0) and general disorders and administrative site conditions (9.5% vs 1.6%) in the isavuconazole arm; and drug-related psychiatric disorders (isavuconazole: 3.2%; voriconazole: 21.0%), eye disorders (3.2% vs 19.4%), and investigation abnormalities (7.9% vs 14.5%) in the voriconazole arm. In the <65 years subgroup, higher proportions of patients (cut-off difference: ≥5%) with drug-related AEs were observed only in the voriconazole arm: investigation abnormalities (isavuconazole: 10.3%; voriconazole: 19.3%), hepatobiliary disorders (2.6% vs 11.7%), psychiatric disorders (2.1% vs 8.1%), and eye disorders (3.1% vs 8.1%).
Efficacy
In VITAL, all-cause mortality through day 42 was higher (30.0% vs 13.8%) and the overall response at EOT was lower (27.6% vs 46.8%) in the ≥65 years than the <65 years subgroup (Table 2).
In SECURE, all-cause mortality through day 42 was similar between both subgroups and both the isavuconazole (20.6% vs 17.9%) and voriconazole (22.6% vs 19.4%) treatment arms. Overall response at EOT was lower in the ≥65 years subgroup of the isavuconazole arm (23.7% vs 39.0%) as compared to the voriconazole arm (32.0% vs 37.5%) (Table 2).
Conclusions: As expected, overall, the safety and efficacy outcomes were more favorable in the <65 years compared to the ≥65 years subgroup in both studies, although, the number of patients was relatively low in the ≥65 years subgroup. In SECURE, all-cause mortality through day 42 was similar between both subgroups and treatment arms, and the safety profile favored isavuconazole in both age subgroups.
Hamed: Basilea Pharmaceutica International Ltd.: Consultancy. Engelhardt: Basilea Pharmaceutica International Ltd.: Current Employment. Kovanda: Astellas Pharma Global Development, Inc.: Current Employment. Huang: Pfizer Inc: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Yan: Pfizer Inc: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Aram: Pfizer Inc: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal